Modular process system
Key words:
Pharmaceutical Engineering
Classification:
Detailed introduction
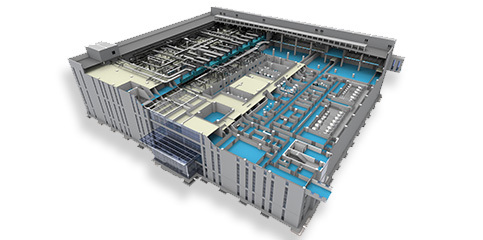
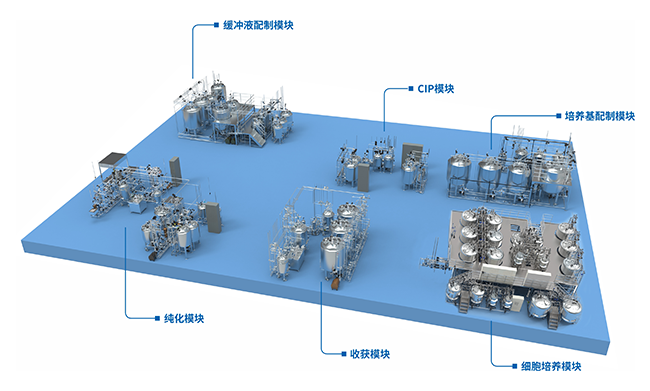
BIO-YD provides users with biological pharmaceutical equipment turnkey solution, meeting the needs of building different production lines, including main process equipment for strain screening, strain expansion, culture/fermentation, harvesting, crude purification, inactivation/detoxification, hydrolysis, purification, formulation preparation/filling; auxiliary process equipment such as medium preparation, buffer preparation, online cleaning system, biological wastewater inactivation system.
The equipment provided by BIO-YD not only meets the technical requirements, process requirements, hygiene requirements, safety requirements, environmental requirements, automation requirements, but also can meet the GMP 2010 revision and related guideline, EU GMP Volume IV, cGMP 21 CFR Part 210 & 211, WHO certification, GB150-2011, ASME BPE 2016 standards and other related specifications according to different needs.
Recommended Products