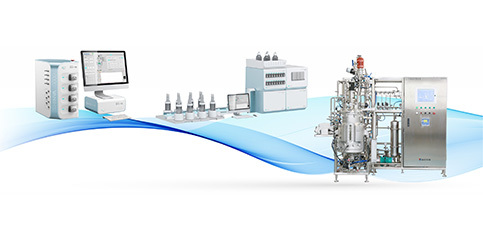
Welcome to the official website of BIO-YD-Pharmaceutical
- Home
-
Designing Institute
Designing Institute
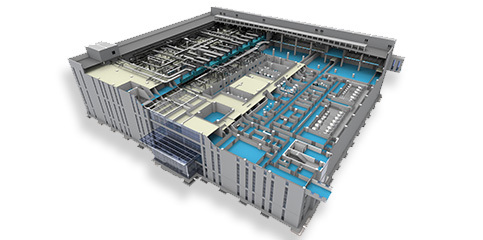
- Process
- Product
- Engineering
- Solutions
- News
- About Us
Key words:
Pharmaceutical Engineering
Classification:
Detailed introduction
Official WeChat

Chengdu Yingde Biomedical Equipment Technology Co., Ltd.
Telephone:028-85925318(switchboard)85925378(Market)
Mailbox:cdyd@bioyd.com
Address: No. 669,Nanliu Road Chengdu Economical Development District Chengdu City, Sichuan Province, China
CopyRight©2023 BIO-YD-Pharmaceutical All rights reserved
SAF Coolest v1.2 设置面板 TDZSX-ZBQV-EXXZE-AFA
修改浏览器滑块样式: 4px,4px,#f00
无数据提示
Sorry, the current column has no content for the time being.!
You can view other columns or returnHome Page