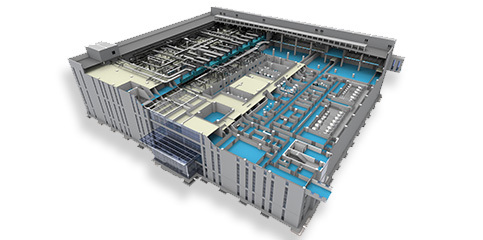
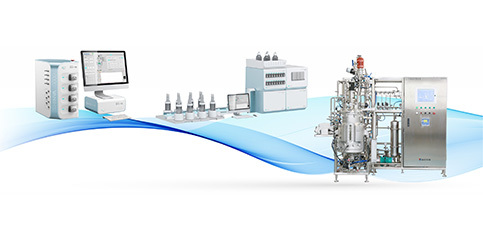
Automatic Control
Complete automation and information solutions provide comprehensive automation services from design, supply, construction and commissioning, FAT and SAT testing, and computer system GMP validation.
Key words:
Pharmaceutical Engineering
Classification:
Detailed introduction
Complete automation and information solutions
Complete automation and information solutions provide comprehensive automation services from design, supply, construction and commissioning, FAT and SAT testing, and computer system GMP validation.
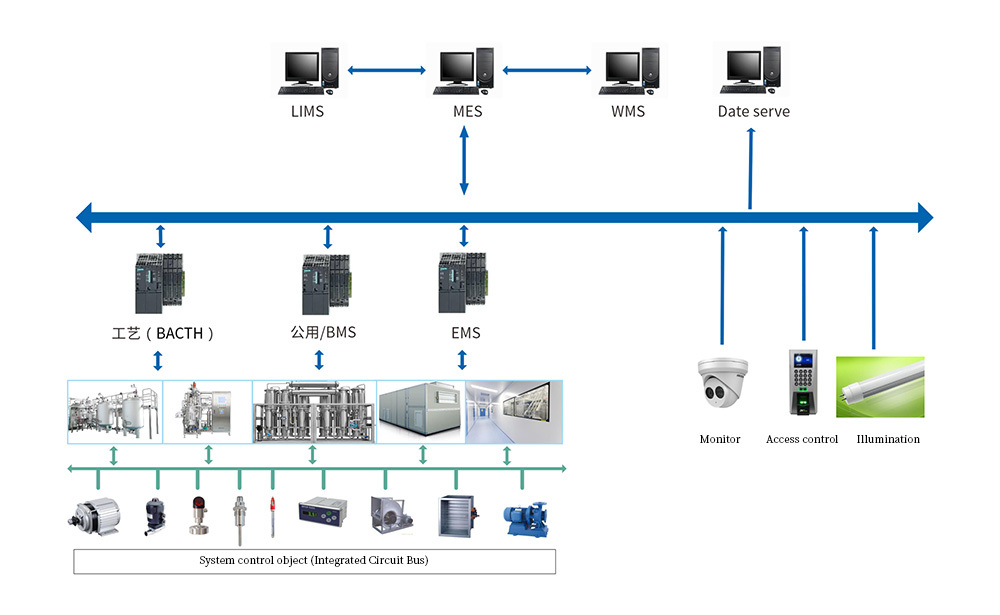
BIO-YD provides pharmaceutical enterprise MES system, SCADA system, BMS system and EMS system, process control system .

Automatic control system validation service
For pharmaceutical companies, automatic control systems require GMP validation. BIO-YD has many years of xperience in the pharmaceutical industry, using the concept of “full life cycle development” to ensure the quality consistency and service integrity of BMS/EMS/SCADA system engineering projects, in line with relevant GMP regulations.
Computer system validation laws and regulations
US, EU regulations
• 21CFRpart11 (Article 21, Section 11 of the US Federal Law)
• 21 CFRpart 211.68.100a (Article 21, Section 21 of the US Federal Law)
• GAMP5
Chinese laws and regulations
• Computerized System of the State Food and Drug Administration
• China GMP
Computer system check main file list
• URS user requirements description
• RA risk assessment
• QPP quality and project plan
• DQ design qualification document
•IQ installation qualification file
• SAT site acceptance test file
•FS function description
• VR validation plan
•DS design instructions
• FAT factory acceptance test file
• OQ operation qualification file
• Vr validation report