- Home
-
Designing Institute
Designing Institute
- Process
- Product
- Engineering
- Solutions
- News
- About Us
Validation


The professional validation team has rich experience and can complete the communication from the communication in early stage, protocol drafting, writing, formulating and implementing the plan. The report is completed and the whole process of verification is provided.
Key words:
Pharmaceutical Engineering
Classification:
Detailed introduction
Validation
The professional validation team has rich experience and can complete the communication from the communication in early stage, protocol drafting, writing, formulating and implementing the plan. The report is completed and the whole process of verification is provided.
Validation of HVAC systems, process systems, automation systems, water systems, clean compress air systems and equipment facilities.
Documents
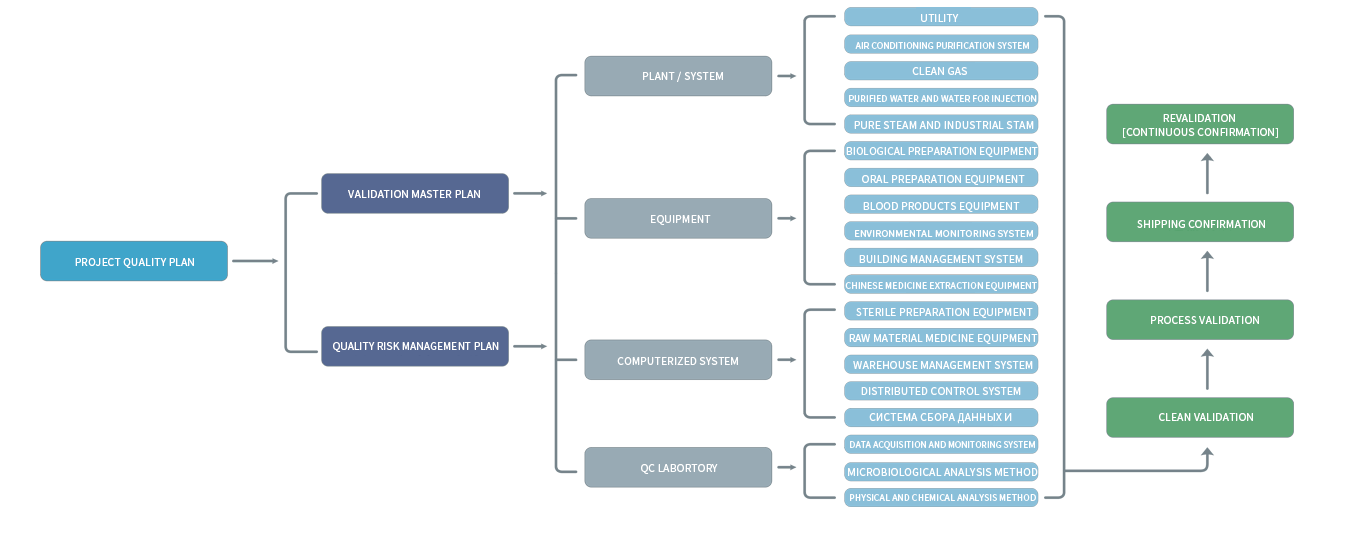
The validation team makes a complete validation protocol and validation plan according to the requirements of the customer, and completes and provides the validation documents during the implementation process. Assist users to pass GMP regulations and validation requirements in China, the US, EU and WHO.

Official WeChat

Chengdu Yingde Biomedical Equipment Technology Co., Ltd.
Telephone:028-85925318(switchboard)85925378(Market)
Mailbox:cdyd@bioyd.com
Address: No. 669,Nanliu Road Chengdu Economical Development District Chengdu City, Sichuan Province, China
CopyRight©2023 BIO-YD-Pharmaceutical All rights reserved
SAF Coolest v1.2 设置面板 TDZSX-ZBQV-EXXZE-AFA
无数据提示
Sorry, the current column has no content for the time being.!
You can view other columns or returnHome Page