Company Profile

Yingde Bio-Pharmaceutical Engineering Overall Solution Service Provider
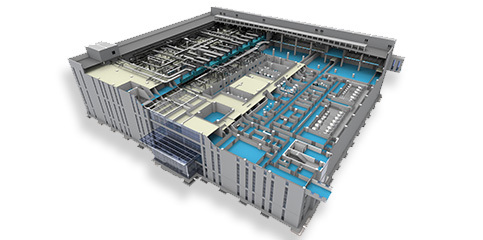
Yingde Bio focuses on pharmaceutical engineering and provides total solutions for the industry
The service areas include: pharmaceutical engineering design and consulting services; research and development, design, manufacturing and verification of high-standard sanitary containers and modular process equipment; pharmaceutical engineering construction and verification.
Joined Xinhua Medical Group in 2014, after the strong integration of Xinhua Medical Pharmaceutical Technology Group, it has developed steadily in recent years and become a leading overall solution service provider for pharmaceutical travel.
Professional design lead
Yingde Bio focuses on the pharmaceutical industry and provides customers with complete pharmaceutical engineering designs that meet their production needs.
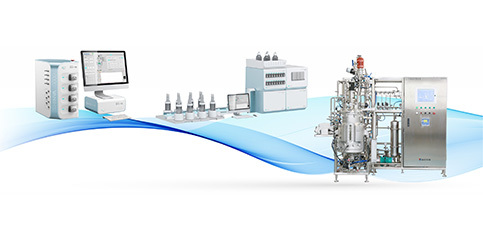
High-end equipment manufacturing
Provide high-standard sanitary containers and accessories for the pharmaceutical industry, and provide design, manufacturing and verification services for modular process equipment to meet different needs.
Overall case resolution
As an EPC service provider, we provide engineering and validation services that meet cGMP requirements.
Memorabilia
1994

1994
In 1994, BIO-YD founded and focused on serving the pharmaceutical engineering industry
2008

2008
In 2008, established a process modular production line and realized the human vaccine process module plant FAT successfully.
2014

2014
In 2014, joined SHINVA group
2017

2017
In 2017,The capacity of the container and process equipment module reaches 1,000 sets per year.
2019
2019
In 2019, the company's output value exceeded 500 million yuan,
Qualifications and Honors

Qualification Of Medical Engineering Design

Qualification Certificate Of Construction Enterprise

Generalunternehmer Der Dritten Ebene Des Maschinen- Und Elektrotechnikbaus

Ce-zertifizierung

Zertifizierung Des Qualitätsmanagementsystems Is09001

Hse-managementsystem (health_safety_environment)

Hse-managementsystem (health_safety_environment)

Hse-managementsystem (health_safety_environment)

Qualifizierung Der Installation Von Druckleitungen

Gc-design Der Druckleitung_installation, Modifikation Und Reparaturqualifikation

Gc-design Der Druckleitung_installation, Modifikation Und Reparaturqualifikation

Asme_u_ Druckbehälterstempel

D1, D2 Druckbehälter Design_manufacturing Qualifikation
After-sales service
Specialized after-sales service department to provide users with professional after-sales service guarantee.
Provide free after-sales consulting services, 24-hour service response, to ensure that the user production on schedule.
We are adhering to the "quality first, customer first" principle of service, continuous development and improvement, to provide users with more quality products and thoughtful service.

Customer Service